All published articles of this journal are available on ScienceDirect.
Preparation and Antioxidant Activity of Purple Potato Wine
Abstract
Purple potatoes were used as raw material to study the purple potato wine production process and antioxidant activity. This paper analyzed different fermentation time, fermentation temperature, yeast inoculum, initial pH, the initial sugar content on alcohol and anthocyanin contents of purple potato wine by single factor experiments and response surface methodology(RSM). The results showed that the optimum fermentation conditions of purple potato wine were as follows: fermentation temperature was 26oC, yeast inoculum was 0.15%, fermentation time was 7 d, initial pH was 3.0 and initial sugar content was 11 %. Under these conditions the alcohol and anthocyanin contents of purple potato wine could reach 10.55%/Vol and 6.42 μg/mL, respectively. The purple potato wine was purple, bright in colour, pleasant fragrance and pure taste. Prepared purple potato wine had the ability of reducing Fe3+ and scavenging superoxide anion radicals, which meant that purple potato wine had certain antioxidant activity.
1. INTRODUCTION
With the improvement of living standards, green, suitable, moderate, healthy way of eating was more and more sought after by people. Drinking high alcohol liquor, such as white spirits, foreign wine, for long time increases the incidence and total mortality for cardiovascular disease and hepatitis disease. Therefore, the low concentration wine with the function of health care is becoming an important part of people's daily diet [1-3].
Purple potato, as a new type of sweet potato varieties, is rich in anthocyanin and selenium. Anthocyanin is a kind of free radical scavengers, which can be beauty, prevention of disease. Selenium is an essential trace element in the human body, which can inhibit and kill cancer cells [4-5]. According to statistics, due to improper preservation, rotten sweet potato can reach to dozens of tons in our country every year, which caused great waste and loss. Thus the development and utilization of purple sweet potato attracting the interest of many people at home and abroad has a broad prospect. In Japan, purple sweet potato wine, with purple sweet potato as raw material, has become a much-welcome healthy drink wine duo to its rich nutrition, low degree of alcohol, soft taste [6]. At present, brewing technology of purple sweet potato wine in China is still in the primary level of research and development. Although there are a few available products, it has not yet become specialized, large-scale industrial production in general [7].
This research studied the preparation of purple sweet potato wine with purple sweet potato as raw material. On the one hand, it provided a new processing way of purple potato, enhanced the added value of sweet potato, and solved the hoarding problems of sweet potato. On the other hand, itadded a new variety of fruit wine. The research also studied the antioxidant activity of purple potato wine to submit some theoretical information and basic data for its mass production and development.
2. MATERIAL AND METHODS
2.1. Materials and Reagents
Purple potatoes were offered by market; Angel Yeast was offered by Angel Yeast Co Ltd.
Glucoamylase (50000 U / g), α-amylase (4000 U /g), citric acid, sucrose etc were food grade additives. Potassium dichromate, ferric chloride, pyrogallol, 95% ethanol etc were analytically pure.
2.2. Methods
Activation of Angel Yeast. Angel Yeast, added to 5.0% sucrose solution, was propagated for 30 min at 35oC with shake cultivation [8]. Then, the strain of Angel Yeast had been obtained and stored at 4oC in the refrigerator for further sutdy.
The preparation of purple potato wine. (1) The fresh purple potatoes without pests and mildew were washed, peeled, and diced into about the size of 100 cm3. (2) The diced potatoes were cooked, and mashed into pasty. (3) The potato paste were mixed into jelly with the ratio of pasty-water at the l:15. (4) The jell was liquefied for 2.5 h at 65oC with 0.15% α-amylase and saccharified for 2.5 h at 60oC with 0.15% glucoamylase. (5) The saccharified material was inoculated to ferment at the different conditions. (6) After filtration and sterilization to the fermented liquid, the product was obtained.
Determination of contents of alcohol and anthocyanin in purple potato wine. The content of alcohol was detected with spectrophotometry [9]. The content of anthocyanin in purple potato wine was detected with the methods of extinction coefficient [10].
Optimization of preparation conditions of purple potato wine. Different conditions, including fermentation time, fermentation temperature, yeast inoculum, initial pH, the initial sugar content were studied by single factor experiments to investigate their effect on the alcohol and anthocyanin contents of purple potato wine. Based on the single factor experiments, the response surface experiments were applied to optimize the preparation conditions of purple sweet potato wine.
Measurement of antioxidant activity of puple potato wine. The reduction effect of puple potato wine on Fe3+ was detected with the methods of ferric-reducing antioxidant power [11]. The scavenging effect of puple potato wine on superoxide anion radicals was detected with the methods of pyrogallol autoxidation [12].
3. RESULTS AND ANALYSIS
3.1. Single Factor Experiments of Preparation Conditions of Puple Potato Wine
Effect of fermentation time on alcohol and anthocyanin contents of purple potato wine. The fermentation time was changed as 4 d, 5 d, 6 d, 7 d, 8 d to investigate their effect on alcohol and anthocyanin contents of purple potato wine. The results were shown at Fig. (1).
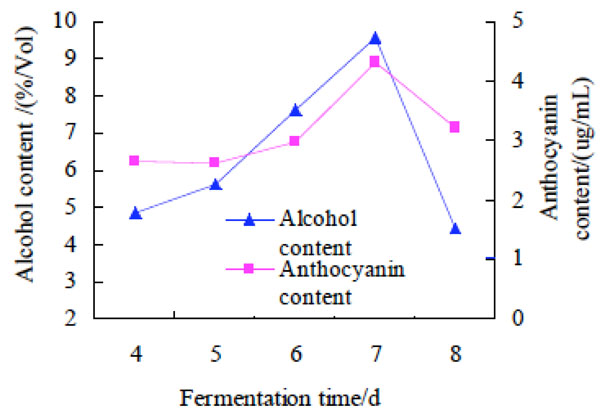
Effect of fermentation time on alcohol and anthocyanin contents of purple potato wine
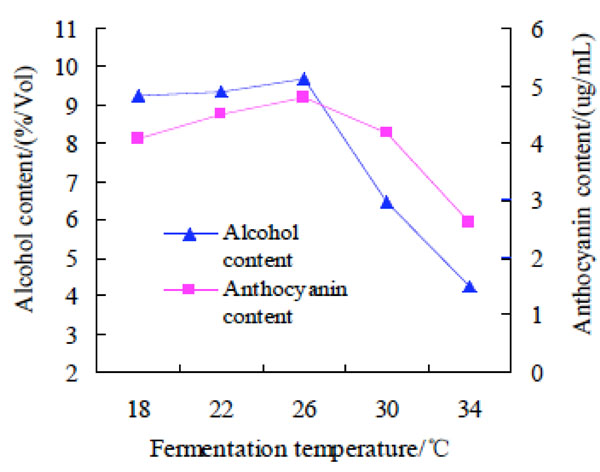
Effect of fermentation temperature on alcohol and anthocyanin contents of purple potato wine
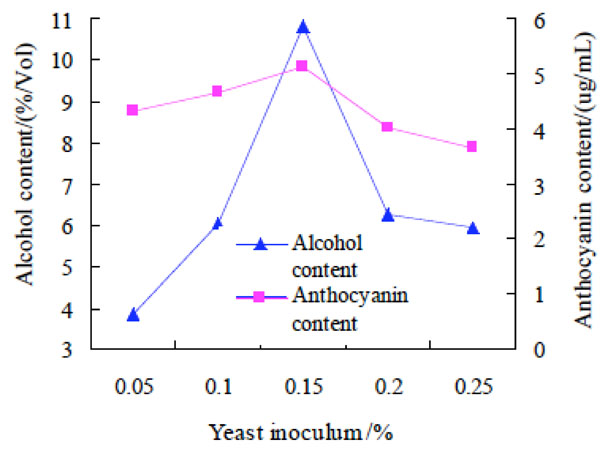
Effect of yeast inoculum on alcohol and anthocyanin contents of purple potato wine.
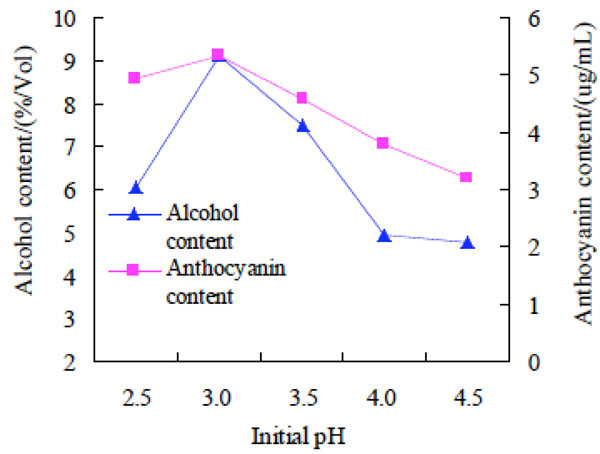
Effect of initial pH on alcohol and anthocyanin contents of purple potato wine.
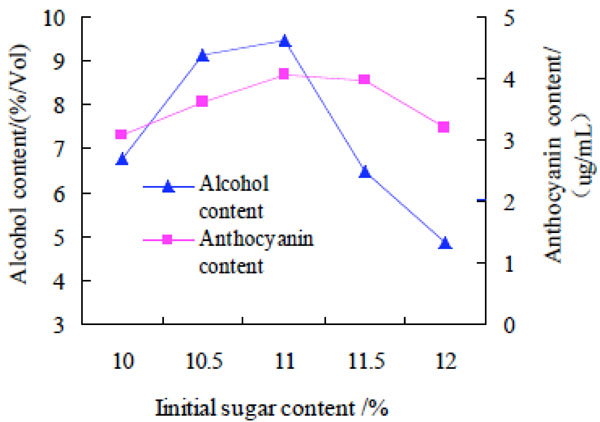
Effect of the initial sugar content on alcohol and anthocyanin contents of purple potato wine.
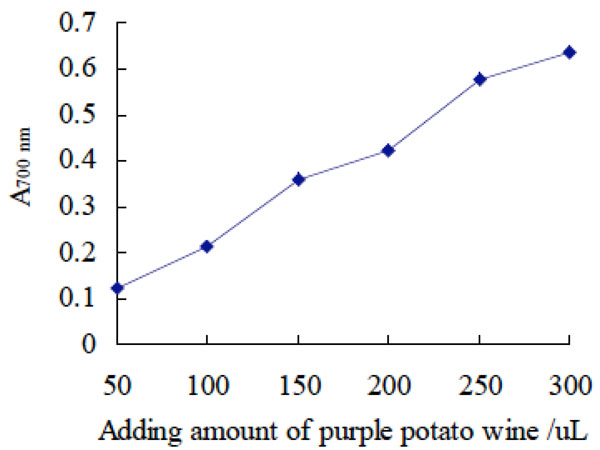
Reduction effect of purple potato wine on ferric iron.
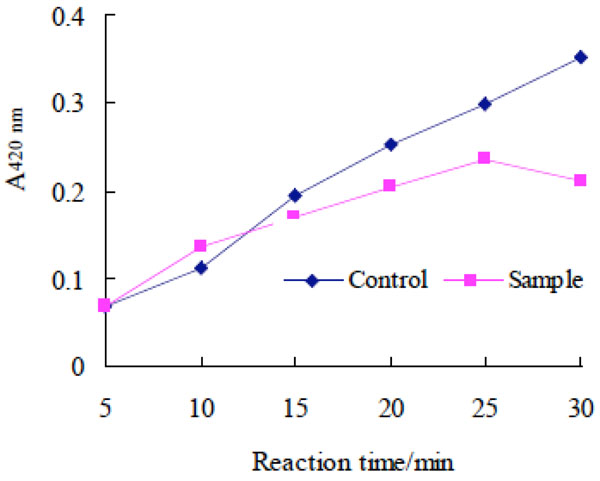
Scavenging effect of purple potato wine on superoxide anion radicals.
Factors and levels of RSM.
| Levels | Factors | ||
|---|---|---|---|
| A Initial pH | B Yeast Inoculum / % | C Fermentation Temperature/ oC | |
| -1 | 2.5 | 0.1 | 22 |
| 0 | 3.0 | 0.15 | 26 |
| +1 | 3.5 | 0.2 | 30 |
Scheme and Results of RSM.
| Experiment Number | A | B | C | Alcohol Content(%/Vol) |
|---|---|---|---|---|
| 1 | 0.00 | 0.00 | 0.00 | 9.942 |
| 2 | 0.00 | 0.00 | 0.00 | 10.091 |
| 3 | 1.00 | 0.00 | -1.00 | 5.545 |
| 4 | 0.00 | 1.00 | -1.00 | 6.786 |
| 5 | 0.00 | 0.00 | 0.00 | 9.884 |
| 6 | 0.00 | 0.00 | 0.00 | 9.765 |
| 7 | 0.00 | 0.00 | 0.00 | 10.346 |
| 8 | 1.00 | 0.00 | 1.00 | 3.542 |
| 9 | 1.00 | 1.00 | 0.00 | 8.600 |
| 10 | 1.00 | -1.00 | 0.00 | 6.653 |
| 11 | -1.00 | -1.00 | 0.00 | 7.894 |
| 12 | -1.00 | 1.00 | 0.00 | 5.433 |
| 13 | 0.00 | -1.00 | -1.00 | 6.785 |
| 14 | -1.00 | 0.00 | 1.00 | 6.854 |
| 15 | 0.00 | -1.00 | 1.00 | 4.865 |
| 16 | 0.00 | 1.00 | 1.00 | 4.875 |
| 17 | -1.00 | 0.00 | -1.00 | 4.879 |
ANOVA for the regression response surfuce model.
| Source | df | Sum of Squares | Mean Square | F value | P value | Significance |
|---|---|---|---|---|---|---|
| Model | 9 | 73.59 | 8.18 | 6.69 | 0.0016 | ** |
| A | 1 | 0.065 | 0.065 | 1.11 | 0.7640 | |
| B | 1 | 0.032 | 0.032 | 0.34 | 0.8336 | |
| C | 1 | 1.86 | 1.86 | 1.48 | 0.1382 | |
| A2 | 1 | 12.77 | 12.77 | 19.21 | 0.0032 | ** |
| B2 | 1 | 5.27 | 5.27 | 7.93 | 0.0259 | * |
| C2 | 1 | 39.40 | 39.40 | 59.24 | 0.0001 | ** |
| AB | 1 | 4.86 | 4.86 | 7.30 | 0.0305 | * |
| AC | 1 | 3.96 | 3.96 | 5.95 | 0.0448 | * |
| BC | 1 | 0.00002 | 0.00002 | 0.00003 | 0.9958 | |
| Residual | 7 | 4.66 | 0.67 | |||
| Lack of Fit | 3 | 4.46 | 1.49 | 29.72 | 0.0034 | * |
| Pure Error | 4 | 0.20 | 0.050 | |||
| Cor Total | 16 | 78.24 |
“**”( P<0.01) was highly significant; “*”( P<0.05) was significant.
It was shown in Fig. (1) that the longer the fermentation time was, the higher the alcohol and anthocyanin contents of purple potato wine was. The alcohol and anthocyanin contents increased to a peak value when the fermentation time was 7 days, but then decreased with the extension of time. The possible reason for the change of alcohol content might be that the fermentation time was less than 7 days, the yeast was in a rapid growth phase and the synthesis of alcohol was less than the peak value, and if the fermentation time was much longer than 7 days, the yeast could consume the sugar in excess and the alcohol content was also low. The possible reason for the change of anthocyanin contents might be that the existent state of anthocyanin was connected with the acidity of purple sweet potato wine determined by fermentation time, and only the polymer anthocyanin could be measured. At 7 days of the fermentation time, results of anthocyanin content reached the maximum, which meant that the amount of anthocyanin in the polymer state was more than others, and the bioactivity of the polymer anthocyanin was also higher [13]. Therefore, the optimal fermentation time was 7 days for preparation of purple potato wine among the experiments.
Effect of fermentation temperature on alcohol and anthocyanin contents of purple potato wine. The fermentation temperature was changed as 18oC, 22oC, 26oC, 30oC, 34oC to investigate their effect on alcohol and anthocyanin contents of purple potato wine. The results were shown at Fig. (2).
It was shown in Fig. (2) that with the increase of fermentation temperature, the contents of alcohol and anthocyanin increased slowly. The alcohol and anthocyanin contents increased to a peak value at 26oC, but then decreased sharply with the raise of temperature. The possible reason for the rapid decrease of alcohol and anthocyanin contents might be that the temperature was too high in yeast to grow and metabolism, leading to premature aging of yeast. The ability to convert sugar into alcohol in the fermentation liquid was reduced, and the alcohol content was less [14].The change of anthocyanin content at different fermentation temperatures might be related to the existent state of anthocyanin. Therefore, the optimal fermentation temperature was 26oC for the preparation of purple potato wine.
Effect of yeast inoculum on alcohol and anthocyanin contents of purple potato wine. Yeast inoculum was 0.05%, 0.10%, 0.15%, 0.20% and 0.25%, respectively, and other experimental conditions fixed, to investigate the effect on alcohol and anthocyanin contents of purple potato wine. The results were shown at Fig. (3).
The results drawn from Fig. (3) was that the content of anthocyanin in purple sweet potato wine changed little with the increase of yeast inoculums, while the change of alcohol content was relatively large. The alcohol content was the highest when the yeast inoculums were 0.15%. The possible reason for the change of alcohol content might be that when yeast inoculum was lower, the sugar of fermentation broth could not be completely converted to alcohol, resulting in less alcohol. when the yeast inoculum was too high, a large number of yeast will consume sugar in fermentation broth to meet its growth and metabolism, so the sugar could not be converted to alcohol, causing the decrease of the alcohol content [15]. Therefore, the optimal yeast inoculum was 0.15% for preparation of purple potato wine.
Effect of initial pH on alcohol and anthocyanin contents of purple potato wine. The initial pH value was 2.5, 3.0, 3.5, 4.0 and 4.5 respectively to investigate the effect on alcohol and anthocyanin contents of purple potato wine. The results were shown in Fig. (4).
The result drawn from Fig. (4) was that the alcohol and anthocyanin contents reached the peak at pH 3.0, and at other pH values, the contents were lower. The possible reason for the change of alcohol and anthocyanin content might be that the growth and metabolism of yeast was inhibited, caused by higher or lower initial pH, which blocked the generation of alcohol, and structure stability of polymeric anthocyanin would be decreased because of higher or lower initial, resulting in the decrease of the anthocyanin content. Therefore, the optimal initial pH value was 3.0 for preparation of purple potato wine.
Effect of the initial sugar content on alcohol and anthocyanin contents of purple potato wine. The initial sugar content was 10%, 10.5%, 11%, 11.5% and 12% respectively, and other experimental conditions fixed, to investigate the effect on alcohol and anthocyanin contents of purple potato wine. The results were shown in Fig. (5).
The result drawn from Fig. (5) was that the change of anthocyanin content in purple sweet potato wine was not great with the increase of the initial sugar content, which were in agreement with Yang Yali's results [12], while the change of alcohol content was relatively large. Alcohol content increased fast with the increasing of the initial sugar content. The alcohol content increased to a peak value when the the initial sugar content was 11%, then the alcohol content decreased rapidly. The possible reason was that sugar could convert into alcohol. When the initial sugar content was not enough, alcohol was less. When the initial sugar content was too much, Yeast would be rapid growth and metabolism, leading to premature aging, and the ability to convert to alcohol in the late stage of fermentation became weak. Therefore, the optimum initial sugar content was11%.
3.2. The Response Surface Experiments of Preparation Conditions of Puple Potato Wine
Based on the principle of Box-Benhnken central composite design, and the results of single factor experiments, the preparation conditions of purple potato wine were optimized by RSM and the factor level of RSM was shown in Table 1. The experiment results of RSM were shown in Table 2 and Table 3.
Based on the test data in Table 2, the effect of the initial pH, yeast inoculum, fermentation temperature on the alcohol content was analyzed with the software of Design Expert 8.0 to do multiple regressions, and the acquired ternary quadratic response surface regression equation was: Y=11.41- 0.42A-0.58B+1.12C-1.24A2-1.36B2-3.97C2+0.65AB-0.70AC+0.81BC.
It was shown in Table 3 that the regression model was highly significant (P < 0.01), and the linear relationship between the dependent variable and examined variable was significant (R2=0.9898). Fitting degree is more than 90%, which indicated that the experimental method achieved the expected effect, and using this equation to simulate the real three factor three level analysis is feasible.
Table 3 showed that the results of variance analysis that the one degree terms of the initial pH (A), yeast inoculum (B) and fermentation temperature (C) was not significant. The quadratic terms A² and C² were highly significant and B² was significant. Interactive effects of initial pH and fermentation temperature, initial pH and yeast inoculum on alcohol contents of purple potato wine were significant, while the interactive effects of yeast inoculum and fermentation temperature was not significant. Thus the relationship of the factors in the experiments was not pure linear while some were nonlinear.
The maximum response values of the regression model predicting the preparation condition of purple potato wine was fermentation temperature at 24.47oC, 3.0 of the initial pH and 0.148% of yeast inoculum. With these conditions, alcohol content reached 10.35%/Vol. Based on the actual operating conditions, the most suitable conditions for the preparation condition of purple potato wine was fermentation temperature at 25oC, 3.0 of the initial pH and 0.15% of yeast inoculum.
To test the reliability of RSM, three parallel experiments were implemented under the most suitable conditions. The average alcohol content was 10.55%/Vol and was little different from the theoretical predicted value, indicating that RSM could be applied to optimize the preparation condition of purple potato wine.
3.3. Antioxidant Activity of Purple Potato Wine
The reduction effect of puple potato wine on Fe3+. Ferric ion was reduced to divalent ions with electrons provided by antioxidants. Solution of divalent ions was light green and has the maximum absorbance at 700 nm. Therefore, antioxidant capacity can be determined on the basis of the absorbance value at 700 nm [16, 17].
It was shown in Fig. (6) that with the increase of adding amount of purple potato wine, the value of absorbance at 700 nm increased gradually, which indicated that purple potato wine had certain antioxidant activity.
The scavenging effect of puple potato wine on superoxide anion radicals. A large amount of oxygen released by pyrogallol autoxidation systems accelerates the rate of pyrogallol autoxidation. In the process above, a series of intermediate product with characteristic absorption in the visible region is generated. Antioxidants added to pyrogallol autoxidation systems can scavenge generated oxygen and inhibit the formation of intermediate product, and therefore the speed of pyrogallol autoxidation becomes slow and the absorbance of the reaction solution at 420 nm decreased. So the antioxidant activity of the tested substances can be evaluated by comparing the absorbance of the reaction solution at 320 nm between sample and control [18].
It was shown in Fig. (7) that compared with the control, the absorbance of sample solution at 320 nm gradually increased slowly with the extension of reaction time, which indicated that the purple potato wine could scavenge uperoxide anion radicals and had certain antioxidant activity.
CONCLUSION
This research studied the preparation and the antioxidant activity of purple potato wine with purple sweet potato as raw material. The results were as follows: The most suitable condition to preparing purple potato wine was: 0.15% of Yeast inoculum, 11% of the initial sugar content, fermentation temperature at 26oC, 7 d of fermentation time, 3.0 of initial pH value. Under these conditions the alcohol and anthocyanin contents of purple potato wine could reach 10.55%/Vol and 6.42 μg/mL, respectively. The purple potato wine was purple, bright in colour, pleasant fragrance and pure taste. Prepared purple potato wine had the ability of reducing Fe3+ and scavenging superoxide anion radicals, which meant that purple potato wine had certain antioxidant activity. That is to say, purple sweet potato wine can prevent the occurrence of the disease, and it is beneficial to people's health. At present, brewing technology of purple sweet potato wine in China is still in the primary level of research and development. Although there are a few available products, it has not yet become specialized, large-scale industrial production in general. Further exploration, research and practice are needed.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work is supported by the Key Project of Scientific and Technological Research of the Education Department of Henan, China (No.13A416110).


