All published articles of this journal are available on ScienceDirect.
Self-Assessment of Biomedical Activity Related to Medical Devices Embedded in EMS Ambulances: Towards a Roadmap for an Efficient Improvement
Abstract
Objective:
The present investigation is focused on a self-assessment of the biomedical activity related to embedded Medical Devices on board a fleet of 46 EMS medicalized ambulances, according to the High Authority of Health standard (criterion 8K) and the Guide of the Good Practices of Biomedical Engineering.
Materials and Methods:
The methodology adopted for this purpose is based on an analysis allowing the evaluation and observation of practices related to biomedical activity in these ambulances. An initial assessment, carried out in March 2021, made it possible to measure the gaps between the actual situation and the recommendations of the two self-diagnosis tools (High Authority of Health and Guide of the Good Practices of Biomedical Engineering standards). A series of corrective actions were proposed and then implemented. A second self-assessment took place after 6 months, in October 2021.
Results:
Between March and October 2021, an improvement in the scores for almost all the axes of the two self-assessment tools was noted. Indeed, the score of the self-assessment for the High Authority of Health reference system rose from 44% in March 2021 to 63% in October 2021, i.e. an increase of 19%, and that of the Guide of the Good Practices of Biomedical Engineering increased from 67.54% in March 2021 to 80.96% in October 2021, i.e. an increase of 13.42%.
Conclusion:
The implementation of a maintenance strategy integrating the notion of quality, relevant procedures and pertinent work tools has made it possible to significantly improve the biomedical activity within the medical ambulances and to optimise the embedded medical devices.
1. INTRODUCTION
Nowadays, health systems in general, and emergency systems in particular, are undergoing major changes. They are seeking to improve the quality of their services and the efficiency of their activities. They must adapt to a rapidly changing context of increasing health care expenditure, scientific and technological progress and changes in user behaviour [1-3].
As a result, the management of medical emergencies is a priority in health system policy. It requires the cooperation of all the actors involved both upstream (pre-hospital emergency medicine) and downstream (hospital emergency medicine) of emergency services [1, 2].
In this context, the Emergency Medical Service (EMS) has a mission of primary importance, namely the regulation of emergency medical assistance from patients’ orientation according to their situation to their-transport destination. The management of medical transport adapted to emergency situations is provided by the EMS’ mobile intensive care units [4, 5].
Thus, factoring in the regulations on the maintenance and quality control of medical devices [6, 7] can lead quite naturally to the implementation of quality procedures. This happens through the establishment of a quality management policy in the exercise of biomedical activity according to standards [8]. For this reason, the main inspiration sources are the professional standards drawn up by biomedical professionals in the form of guides [9-11], as well as or international and European standards [12-14], which are mainly concerned with the management of medical devices [15].
Indeed, ensuring the quality and safety of care is one of the major challenges for any health care institution. In this perspective, the proper management of medical devices is a key element. Promoting quality within one's establishment guarantees a service of good quality [16].
The quality approach fits perfectly in the increasingly normative and regulated world our health care institutions are confronted with. Among other things, it enables them to meet patients' expectations, promote the principle of continuous improvement and strengthen their leadership. Numerous guidelines offer a complete overview of the important elements to be mastered [7, 9, 12, 17, 18].
To date, few authors have focused on the biomedical activity related to the main tool of the EMS missions, namely the mobile intensive care units, or medicalised ambulance [19, 20]. It must be considered as a medical resuscitation device whose reliability and availability are a necessary to guarantee the safety and quality of pre-hospital care [21].
In this paper, we intend to carry out a self-assessment of the biomedical activity related to medical devices on board a fleet of 46 EMS medical ambulances in the Moroccan context. That process was done in two phases: in March 2021 and after 6 months in October 2021. This follows the government's policy to strengthen the regulatory framework for medical devices [22] and health transport.
2. MATERIALS AND METHODS
The present study is performed in the EMS and focuses on the biomedical section activities, more specifically, the medical devices embedded in a fleet of 46 EMS medical ambulances.
The methodology adopted for this purpose is based on an analysis allowing the evaluation and observation of practices related to biomedical activity in these ambulances.
A synergy between the High Authority of Health referential (Criterion 8k) [7] and the Guide of Best Practices in Biomedical Engineering referential [6] was made, and a self-diagnosis based on these two standards was performed.
2.1. Self-Assessment Process
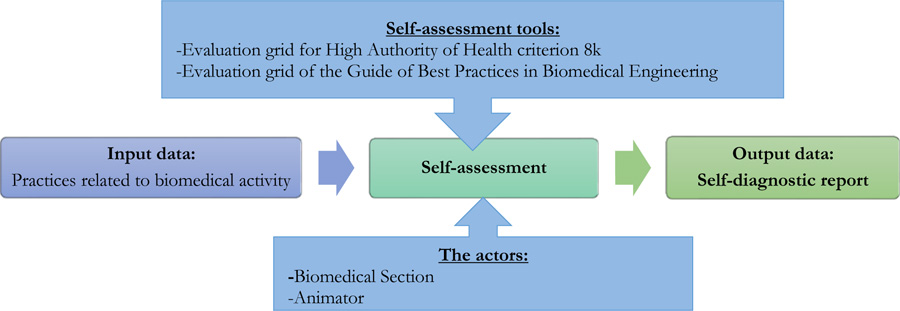
The self-assessment process within the EMS allows the analysis of weak points and identification of actions to be implemented in order to control the management of medical devices and to highlight the improvement actions to be carried out at the end of this process. That process was done in two phases: in March 2021 and after 6 months in October 2021. The process diagram in Fig. (1) shows how the Self-assessment procedure is carried out using the two evaluation grids.
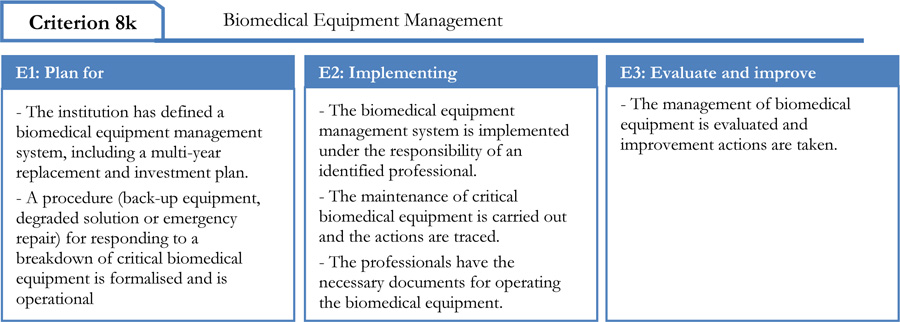
2.2. Criterion 8k of the High Authority of Health Standard
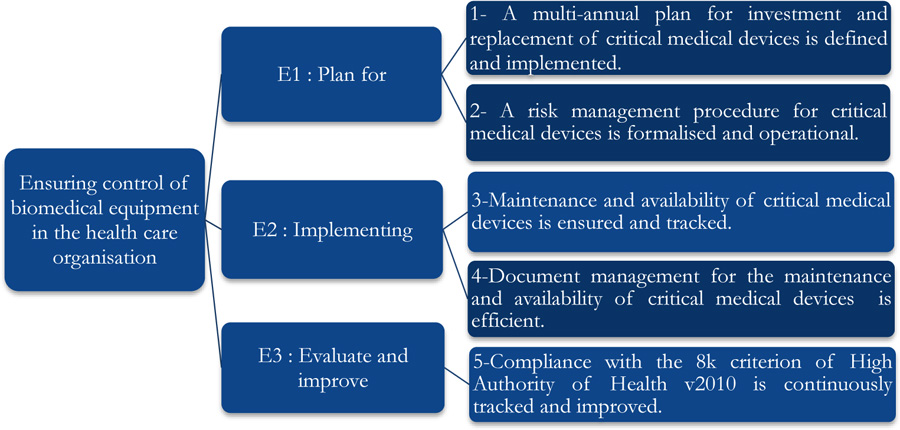
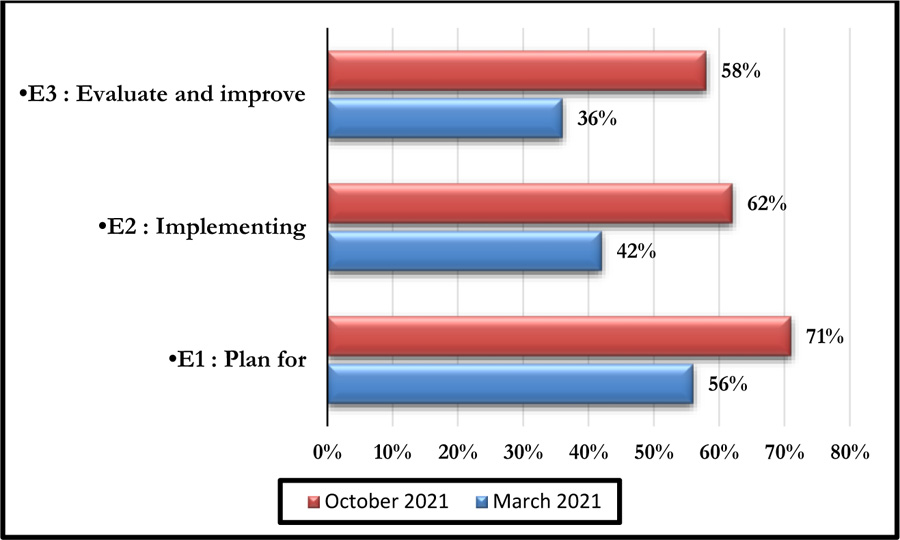
This tool consists of five processes comprising 29 clear iterations. Each of these iterations corresponds to a High Authority of Health assessment level of “E1”, “E2” and “E3” as shown in Fig. (2) [23].
The evaluation grid for criterion 8k of the High Authority of Health reference framework is used to generate the evaluation results by mapping the various items to be evaluated. The evaluation of this criteria is done according to 6 levels of veracity as indicated in Table 1 [7].
| Legends | Scales used in the calculations | ||
|---|---|---|---|
| Item | Rating | % of Veracity | |
| No one in the service has any doubts | False Unanimous | 0 | 0% |
| At least one person considers that the statement is not really false | False | 0.2 | 20% |
| There is nothing to identify the achievement of the action | Somewhat False | 0.4 | 40% |
| The action is carried out randomly | Somewhat True | 0.6 | 60% |
| The action is carried out systematically | True | 0.8 | 80% |
| It is possible to prove to an external peer that the action was carried out | True Proven | 1 | 100% |
Each iteration was assigned a level of veracity expressed as a percentage. This made it possible to give a step-by-step assessment to be able to capitalise on the experience at the end of the procedure. The result was generated in mapping on the High Authority of Health levels and processes.
2.3. The Guide to Best Biomedical Engineering Practice
The Guide to Best Biomedical Engineering Practice (GBBEP) is the most appropriate reference framework for biomedical activity [24] because it is easy to implement. Its main objectives are:
- Continuous improvement and maintenance of sustainable performance of biomedical services;
- Ensuring performance measures: effectiveness, efficiency, perceived quality;
- Self-assessment of biomedical activities.
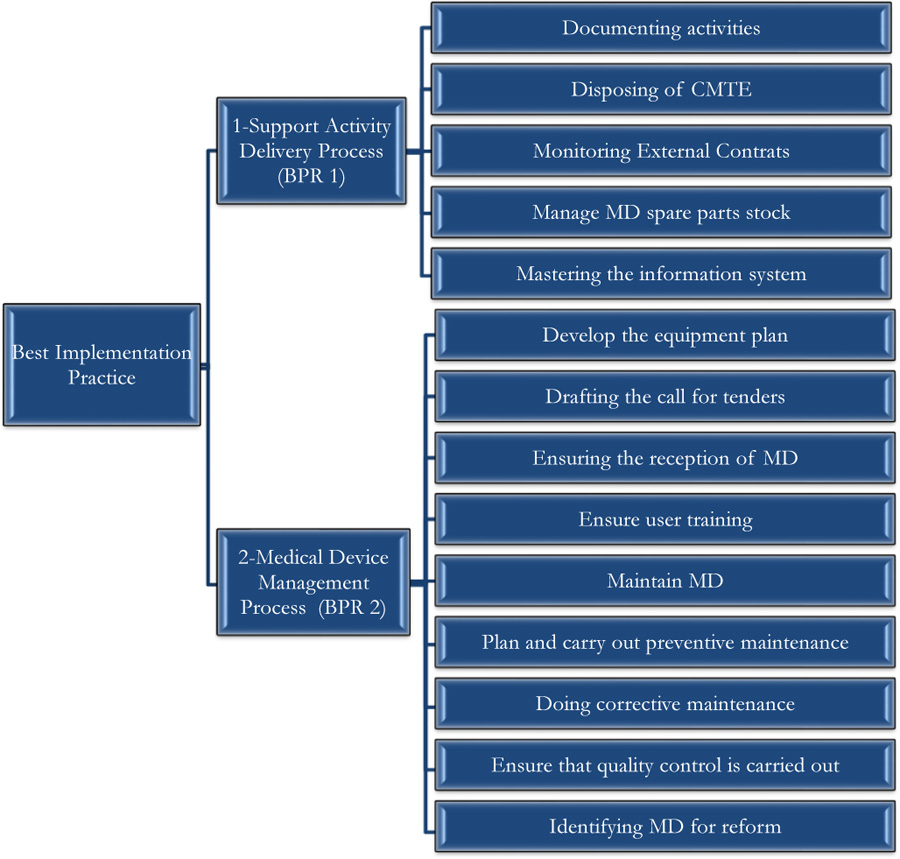
The self-assessment concerned two processes, namely: the Support Activity Process and the Medical Device Management Process.
It was carried out according to the four levels shown in Table 2 [9]. Each statement is assigned a weighting coefficient to distinguish its relative importance within the reference evaluated.
| Statement | Rating | Meaning |
|---|---|---|
| True | 1 | -Staff has easy access to documents, whether they are in paper or digital format. |
| Somewhat true | 0.7 | -Staff has easy access to documents, but they are not validated or incomplete. |
| Somewhat false | 0.3 | -Staff has easy access to documents, but they are not validated or incomplete. |
| False | 0 | - Staff do not have easy access to documents. |
| N/A | - Not applicable in the department concerned. | |
3. RESULTS
3.1. Evaluation on the 8k Criteria of the High Authority of Health v2010
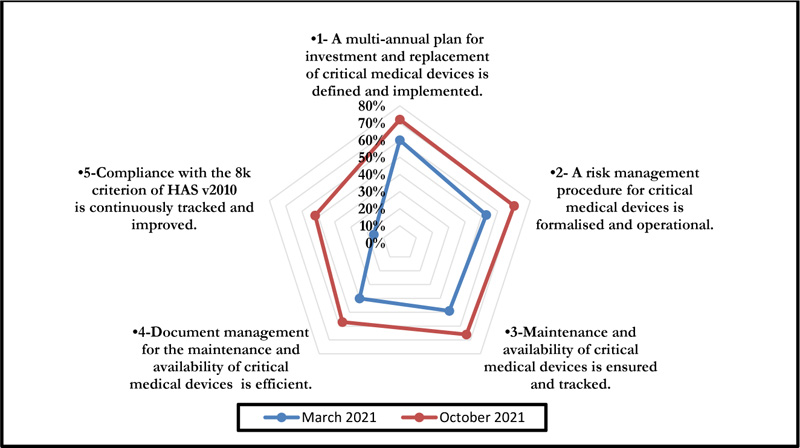
The self-assessment on criterion 8k of the High Authority of Health v2010 standard concerned five axes associated with the E1, E2, and E3 phases and evaluation levels. The positioning of the biomedical section is shown in Fig. (3), and a summarized evaluation grid is presented in Appendix 1.
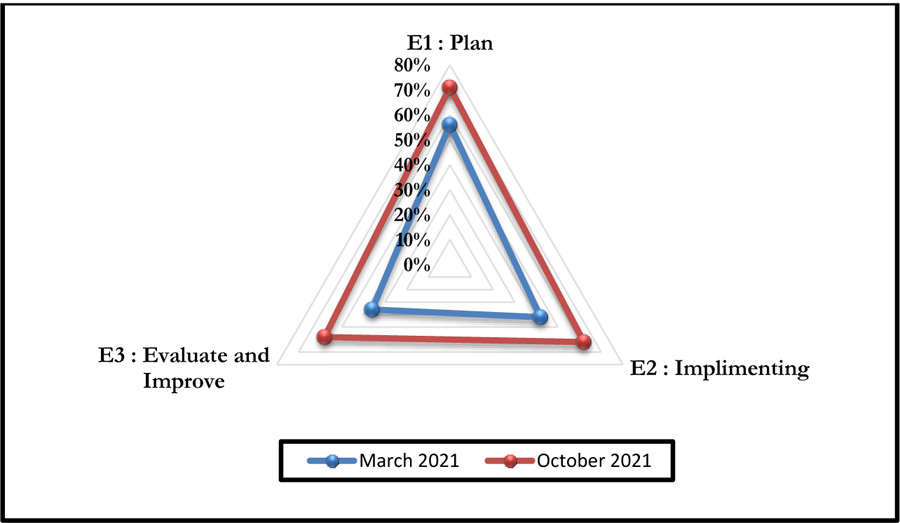
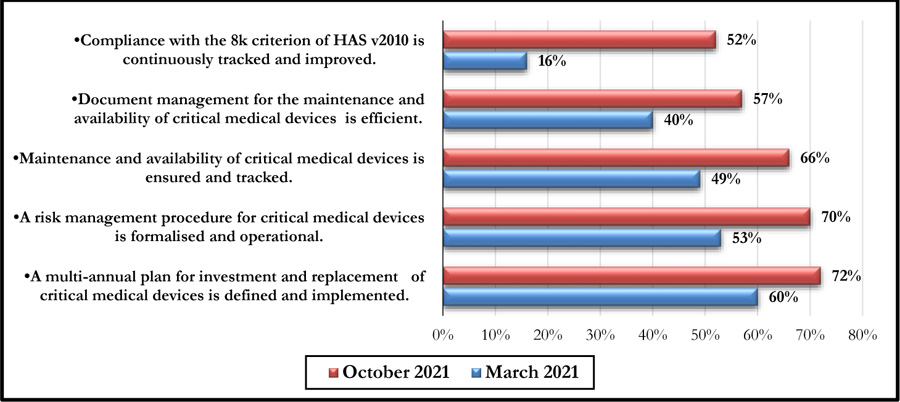
This self-assessment made it possible to obtain a percentage of compliance with the said criteria. Figs. (4 and 5) represent the compliance with High Authority of Health v2010 criterion 8k respectively according to levels E1, E2, and E3 and the rates of veracity of achievement of the process objectives.
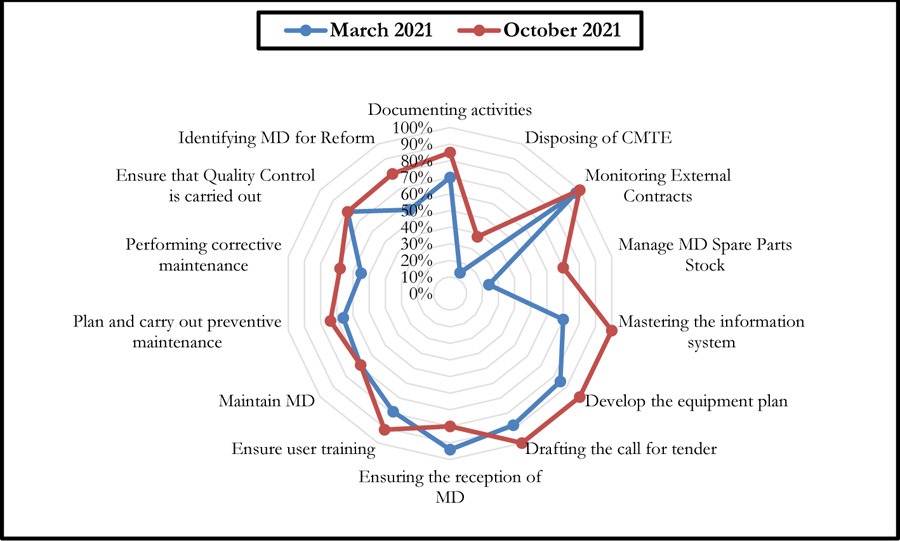
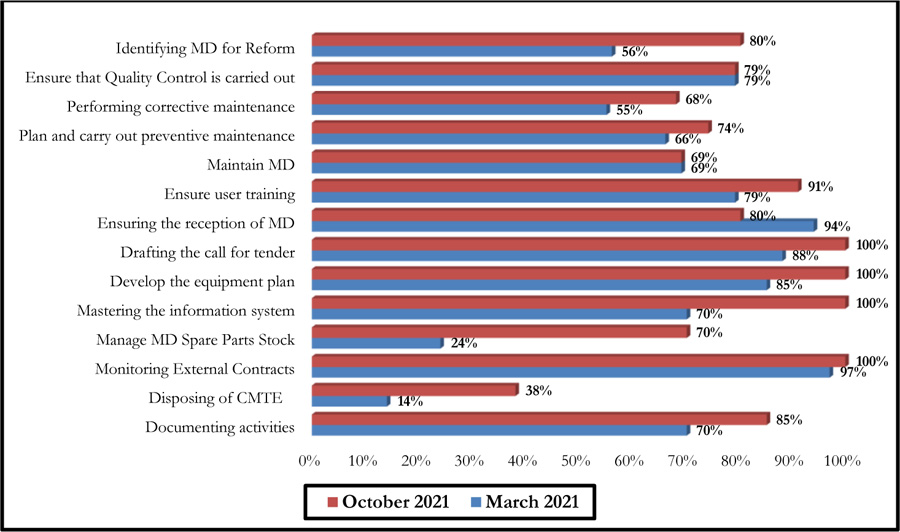
The October 2021 self-assessment achieved scores almost 20% higher than those of March 2021. This improvement concerned the 3 HAS levels as well as the 05 processes (Figs. 6 and 7).
3.2. Evaluation of the Best Practice of the G.B.P.B.E
The self-assessment by the Guide of Best Practice of Biomedical Engineering was made on the good practices of realization since it constitutes the core business of the biomedical activity. Thus, the self-diagnosis concerned the two processes, namely: the Support Activity Process and the Medical Device Management Process, as shown in Fig. (8). A summary of the evaluation grid is presented in Appendix 2.
Fig. (9) summarises the results of this self-assessment in the form of a radar graph.
These graphs give a clear picture of the evolution of the different axes after six months following the first evaluation performed in March 2021. There has been a considerable improvement in the scores of some axes, even reaching the 100% threshold. However, a 14% decrease was noted for the axis entitled: Ensuring the reception of Medical Devices (Fig. 10).
4. DISCUSSION
The self-assessment technique is a natural part of a continuous improvement cycle. Thus, the two self-assessment tools, which are intended to be complementary, have made it possible to highlight the main areas on which the institution must concentrate its efforts. The aim is not only to improve biomedical activities but also to promote the professionalism of the biomedical section.
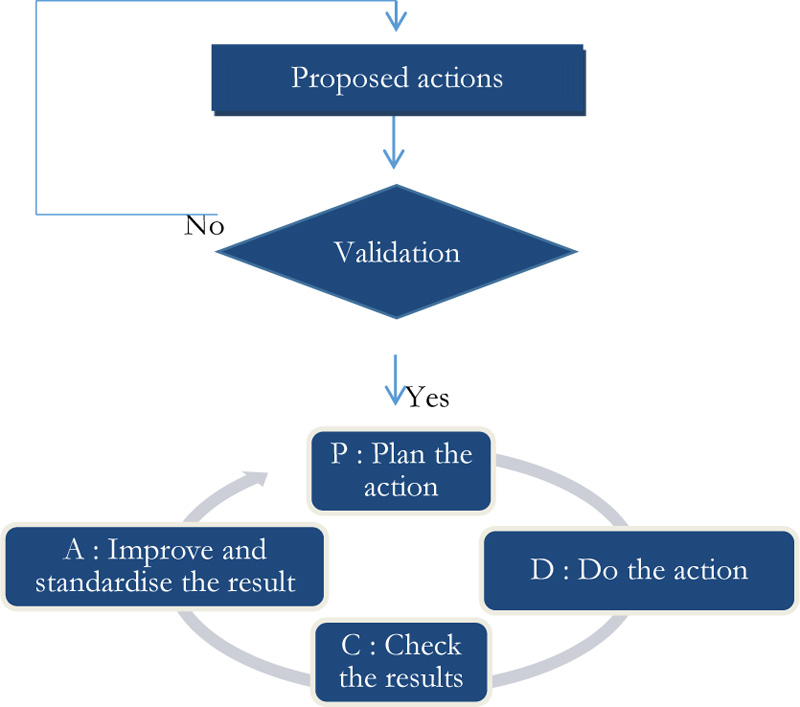
Indeed, the implementation of corrective actions, following deviations from the set recommendations, will follow the Deming PDCA cycle (Fig. 11).
The results obtained following the self-assessment by the HAS (March 2021: 44% / October 2021: 63%) and by the G.B.P.B.E (March 2021: 67.54% / October 2021: 80.96%), show a real determination by the institution to improve the quality of pre-hospital care. It should also be noted that these results remain acceptable given the number of services provided to patients according to the standards in use. Thus, the increase in the scores recorded after six months shows the institution's willingness to keep up with technological developments and to adapt to changes in emergency systems.
Indeed, the effectiveness of the two self-diagnosis tools was reflected in the recommendations on which the improvement efforts were focused over a period of six months. Thus, a synergy between the biomedical department and the contracted company, which provided the necessary resources, allowed for reactivity in working on the recommendations generated. This led to an improvement in the scores for the majority of the axes of the two self-diagnosis tools. A decrease was noted for one of the G.B.P.B.E. axes (Ensuring the reception of medical devices), which is essentially due to the decrease in the purchase of medical devices during this period, which was essentially devoted to the preparation of annual investment plans. It should also be noted that the change in the establishment's strategy led to a change in investments relating to new medical devices, which decreased compared to previous years.
Thus, the framework followed by the biomedical service constituted a real dashboard by centralising and making accessible the data relating to the activity of maintenance, control and management of the biomedical equipment of medical ambulances using the following recommendations:
- Identification of critical medical devices internally with an appropriate method (FMECA) [25, 26];
- Management of risks related to medical devices through the creation of emergency procedures to meet the needs of health care teams;
- Implementation of a dedicated digital platform specifically for the inventory and management of biomedical equipment;
- Provide a schedule for preventive maintenance (forecast or systematic) and control of medical devices embedded in medical ambulances. The periodicity will be fixed according to the type of device and the frequency of use;
- Implementation of the Safety, Quality and Maintenance Register (SQMR) in paper format to encourage the involvement of all persons likely to fill it in and to ensure that it is updated in the digital platform;
- Corrective actions should be subject to consultation of the SQMR to determine whether medical devices have recurring failures;
- Provide for Control, Measurement and Test Equipment (CMTE) for calibration or repair of medical devices;
- Implementation of a dashboard on the maintenance and availability of medical devices embedded in medical ambulances;
- Provide for standardised procedures for quality control and maintenance of medical devices;
- Establish peer-to-peer self-assessment schedules in the future;
- Involve the top level in the results of self-assessments;
- Provide access to documentation relating to biomedical activity in numeric format by the digital platform to ensure speed and guarantee access to other users;
- Updating of documentation related to the biomedical activity and removal of outdated versions;
- Informing users of faults that occur and training them in good usage practices.
CONCLUSION
In this work, we have attempted to emphasise the important and necessary place of maintenance and control of embedded medical devices in EMS ambulances.
This strategy has allowed the identification of actions to be carried out and tools to be used, through the implementation of relevant procedures and working tools that will subsequently contribute to the quality assurance process.
On this basis, it would be interesting to set up a maintenance and quality control strategy as a first step and then to start a global quality approach integrating the managerial aspects of the different institution departments.
Indeed, the two self-assessment tools are complementary and effective solutions available to the biomedical community. They allow continuous improvement and are therefore a prerequisite for any successful maintenance and quality control strategy .
Nevertheless, and in the light of the above, it should be noted that the culture of biomedical activity remains a marginalised concept with a vague outline in Moroccan legislation. In this context, it would be judicious to concentrate efforts in order to regulate biomedical practices, which are the cornerstone of the reliability and safety of biomedical equipment, with the aim of ensuring the quality of patient care. Thus, the development of a Moroccan Accreditation Reference System (MARS) could constitute a real instrument of continuous improvement for our health establishments in their quest for efficiency.
LIST OF ABBREVIATIONS
| CMTE | = Control, Measurement and Test Equipment |
| EMS | = Emergency Medical Service |
| FMECA | = Failure Modes, Effects and Criticality Analysis |
| GBPBE | = Guide of Best Practices in Biomedical Engineering |
| HAS | = French High Authority for Health |
| MD | = Medical Device |
| SQMR | = Safety, Quality and Maintenance Register |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies based on this research.
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
| Action Plans and Iterations | Levels | |||||
|---|---|---|---|---|---|---|
| 1) A multi-annual investment and replacement plan for critical medical devices is defined and implemented | ||||||
| 1.1 | Investments in critical medical devices are identified | E1 | ||||
| 1.2 | Replacements to be made on critical medical devices are identified | E1 | ||||
| 1.3 | The objectives of the multi-annual investment and replacement plans are made explicit and measurable | E1 | ||||
| 1.4 | The process for achieving the above objectives is defined and communicated | E1 | ||||
| 1.5 | Multi-annual investment and replacement plans are assessed annually | E3 | ||||
| 2) A risk management procedure for critical medical devices is formalised and operational | ||||||
| 2.1 | Critical medical devices and their risks are identified according to regulations and uses | E1 | ||||
| 2.2 | Material vigilance, regulatory developments, normative documents and manufacturer data are taken into account | E1 | ||||
| 2.3 | A Safety, Quality and Maintenance Register (RSQM, standard NF S 99-171) is created and available | E1 | ||||
| 2.4 | Back-up, emergency or degraded solution procedures for critical medical devices are formalised and validated | E1 | ||||
| 2.5 | The human and logistical resources required for the above procedures are identified and operational | E1 | ||||
| 2.6 | Risk management procedures for critical medical devices are reviewed annually | E3 | ||||
| 3) Maintenance and availability of critical medical devices is ensured and documented | ||||||
| 3.1 | Maintenance and quality control of critical medical devices are communicated and assured | E2 | ||||
| 3.2 | The Safety, Quality and Maintenance Register (SQMR, standard NF S 99-171) is systematically used | E2 | ||||
| 3.3 | Control, Measurement and Test Equipment (CMTE) is available, calibrated and software is updated | E2 | ||||
| 3.4 | The training required for technical staff is identified and the training taken is recorded | E2 | ||||
| 3.5 | The training required for health care staff is identified and the training taken is recorded | E2 | ||||
| 3.6 | A scorecard on the maintenance and availability of critical medical devices is developed | E2 | ||||
| 3.7 | Improvements on maintenance and availability of medical devices are proposed once a year | E3 | ||||
| 4) Document management for the maintenance and availability of critical medical devices is efficient | ||||||
| 4.1 | User access to instructions for use of critical medical devices is ensured | E2 | ||||
| 4.2 | The Safety, Quality and Maintenance Register (SQMR, standard NF S 99-171) is operational and allows the traceability of actions carried out | E2 | ||||
| 4.3 | Maintenance, quality control, loan and emergency documents are updated, indexed, available and accessible | E2 | ||||
| 4.4 | Regulatory and material safety texts are updated, indexed, available and accessible | E2 | ||||
| 4.5 | Standards relevant to the operation of medical devices are identified, available and accessible |
E2 | ||||
| 4.6 | The quality of document management is assessed once a year and improvements are proposed | E3 | ||||
| 5) Compliance with High Authority of Health v2010 criterion 8k is tracked and continuously improved | ||||||
| 5.1 | Self-assessments on the process “management of biomedical equipment according to criterion 8k” are carried out at least once a year | E3 | ||||
| 5.2 | Reviews of previous sub-processes are carried out and taken into account | E3 | ||||
| 5.3 | Improvements to the process “management of biomedical equipment according to 8k criteria” are proposed | E3 | ||||
| 5.4 | Declarations of conformity between peers or according to standards (ISO 17050) are sought | E3 | ||||
| 5.5 | The Management is informed about the assessment of the process “management of biomedical equipment according to High Authority of Health v2010 criterion 8k”. | E3 | ||||
| BPR-1-01: Documenting activities |
| Staff have easy access to documents, whether in paper or digital format |
| Specific or mandatory (regulatory) documents for the health care organisation or guardians are kept up to date and easily accessible. |
| The process of managing, updating, saving and removing out-of-date versions of biomedical documentation is known to staff and controlled... |
| BPR-1-02: Disposal of Control, Measurement and Test Equipment (CMTE) |
| The biomedical department has the following Control, Measurement and Test Equipment (CMTE) |
| Biomedical technicians are all capable of using Control, Measurement and Test Equipment (CMTE) |
| The list, technical manuals and instructions for use of the equipment and Control, Measurement and Test Equipment (CMTE) of the biomedical service are filed and kept in an identified place so that they can be easily consulted |
| The Biomedical Service's Control, Measurement and Test Equipment (CMTE) are well calibrated and the calibration results are available to all |
| BPR-1-03: Monitoring External Contracts |
| They identify the sectors and areas of activity in which they cannot fulfil their missions and objectives in part or in full with their internal resources and support |
| They negotiate and develop service contracts, based on allocated budgets and desired availability, quality and safety objectives for the operation of medical devices |
| They involve the outsourcing provider, or co-sourcing partner, in the design of performance indicators to ensure the effectiveness of outsourced activities |
| They evaluate the effectiveness of the co-processed, subcontracted or outsourced activities periodically and at least before each contract renewal... |
| BPR-1-04: Managing MD Spare Parts Stock |
| It defines and documents a procurement policy with explicit and known quality criteria |
| Maintains an up-to-date inventory status to control supplies, detect problems and assess their financial and technical value |
| Stored items are labelled and stored in such a way that their quality cannot be affected |
| Stored items with expiry dates can be easily identified. |
| BPR-1-05: Mastering the information system |
| All biomedical staff share a digital (or paper, for lack of a better term) information space, where the inventory of medical devices and useful data for biomedical engineering activities are capitalised |
| Task forecasting and traceability of biomedical engineering actions is ensured and communicated to stakeholders |
| a) The process of backing up, duplicating, archiving and maintaining the durability of the shared information system is implemented periodically and controlled in conjunction with the IT managers. |
| BPR-2-01: Develop the equipment plan |
| They advise, identify and develop, together with the care, medico-technical or technology-user departments and the management, the needs, priorities, justifications and essential characteristics desired in the operation of medical devices in the short, medium and long term |
| They propose to management strategic plans for the investment of new medical devices and the replacement of the most obsolete or critical ones (possibly according to the frequency of breakdowns and the lifespan of the equipment given by the manufacturer) by presenting the implications and effects of their operation |
| They keep a written record of the different phases of the process leading to the funding decision by the management... |
| BPR-2-02: Drafting the call for tender |
| They prioritise potential acquisitions with the care services and management, according to the clinical, technical, logistical and financial impacts they will have in operation, and contribute to the smooth running of the purchasing process |
| They negotiate short-term warranty and, where appropriate, medium-term maintenance contracts at the time of purchase, with the aim of obtaining maximum performance from the medical devices over their expected lifetime |
| BPR-2-03: Ensure the reception of MD |
| It ensures that the regulatory aspects and the constraints of implementation are respected in the layout of the operating sites planned for the installation of medical devices, whether they are fixed or mobile, in the health care institution or, when this is part of its mission, in the patient's home |
| He records information to ensure that what is received conforms to the order in a medical device receipt document |
| If necessary, it carries out functionality tests and measurements of initial performance characteristics called “acceptance tests” or “qualifications” (installation qualification, functional qualification, performance qualification), which are useful in the event of subsequent deviations or complaints |
| In the event of non-conformity identified during acceptance, appropriate measures are taken. Non-conformities are recorded and the means and solutions used to restore conformity are monitored |
| It manages the receipt signed by the parties, documents the inventory, identifies and uniquely marks the devices and informs the relevant care services and directorates... |
| BPR-2-04: Ensure user training |
| It plans, or helps the care and medico-technical services or technology users to plan the user training planned when the medical devices were purchased, and those that become necessary during their operation following staff rotations or changes, as well as the updating of knowledge due to the passage of time or modifications made to the equipment during its operation |
| Ensures at the time of commissioning of the equipment that user guides, instruction, cleaning and operating manuals, written in the language customary in the country and/or the user, are provided and made available to the user personnel |
| He/she shall ensure that the periodic maintenance, calibration and commissioning documentation is available and accessible to any user or other personnel involved when the equipment is first made available |
| It ensures that user training is provided by qualified persons who are competent in relation to the medical devices concerned. It ensures that regulatory training, when it exists, is carried out |
| It records in a document of fitness for use that the medical users and those responsible for care and medico-technical services or users of the technologies have been trained, as required, in the commissioning and handling of the medical device and that they have the documentation useful for their operation and the protocols for verification before their use on patients... |
| BPR-2-05: Maintaining MD |
| It lists devices that are critical to the patient and user, consistent with any regulations or requirements that may exist |
| The criticality is qualified (formulated) but also quantified on a homogeneous scale between 0% and 100% in order to allow comparison between departments and between institutions |
| It provides, with the care, medico-technical or technology user departments of the establishment, a specific process for operating critical medical devices and alternatives or “degraded modes” in the event of a problem |
| He/she ensures the quality and conformity of accessories, consumables and fluids associated with the operation of medical devices |
| It contributes to the vigilance process within the establishment for any potential hazard or operational risk that may be identified |
| He/she ensures that training is provided and that the specific technical means necessary to maintain performance are available to the biomedical service personnel |
| Maintains active communication with health care services on the operation of medical devices and their success criteria in use |
| He/she fills in the inventory and monitoring of medical devices with operating observations and indicators for monitoring success in use |
| It checks that the equipment lent, made available or hired is traced and managed in accordance with the regulations in force |
| BPR-2-06: Planning and carrying out Preventive Maintenance |
| He/she draws up the preventive maintenance programmes according to the manufacturers' data (including any modifications to be made, requested or proposed by the manufacturer), but also taking into account the criticality of the medical device, the regulations, the conditions of use and its intensity of use, and the establishment's maintenance policy validated by the authorities |
| It plans with the care and medico-technical services or technology users the availability of access to medical devices and possibly proposes alternative uses |
| Carries out, or has carried out, preventive maintenance at least in accordance with the manufacturer's recommendations, contractual agreements or validated internal procedures |
| Once the preventive maintenance has been completed, a complete quality control of all the functionalities is carried out, both qualitatively (acceptable, good, excellent, etc.) and quantitatively on the essential characteristics (measurement values and uncertainties) |
| He/she returns the medical devices that comply with quality control to operation, informing the user of the activity carried out and the planned period for the next preventive maintenance (possibly with a label such as “maintenance carried out on: ...”, “next maintenance planned on:...”) |
| BPR-2-07: Performing corrective maintenance |
| It ensures that all users are aware of and use the procedure in place in the event of a medical device failure |
| It plans with the care services the availability of access to the medical devices in breakdown and possibly proposes alternatives of use |
| It sends a competent person from the biomedical department to systematically carry out a fault diagnosis and identify the corrective actions to be taken, if necessary with the help of the maintenance contracts covering the devices |
| Ensures the application of decontamination or disinfection or sterilisation protocols for devices before maintenance |
| It provides user personnel with information on the progress of the corrective maintenance process |
| Once the corrective maintenance has been completed, it carries out a complete quality control of all the functionalities, both qualitatively (acceptable, good, excellent, etc.) and quantitatively on the essential characteristics (values and uncertainties of the measurements) |
| He/she returns medical devices that comply with quality control to operation, informing the user of the source of the failure and the activity performed |
| He/she documents and makes easily accessible to any authorised person a corrective maintenance intervention report containing relevant information (dates and participants, identification of medical devices, technical activities carried out, parts changed and labour time, deviations, defects, malfunctions corrected, tests and checks carried out and their conformity, probable origins of the failure, possible proposals for preventive action, etc.). |
| It ensures compliance with the regulations on the storage of technical data on medical devices |
| It ensures that the confidentiality of data concerning providers is respected... |
| BPR-2-08: Ensure Quality Control is carried out |
| It designs and plans quality control operations |
| It communicates its planning to the services in charge of quality (National Quality Control Service) |
| The device is immediately labelled “compliant” or “non-compliant” depending on the results. A non-conformity is due to a specified requirement not being met |
| He returns to operation the medical devices declared to be in conformity with the quality control, informing the user of the results obtained and the forecast period for the next quality control. He sends back for maintenance the medical devices declared “not in conformity” with the quality control |
| He/she documents and makes easily accessible to any authorised person a quality control report containing the relevant information (date of control carried out and date of the previous control, person involved and authorisation, medical device concerned, ECME used and period of validity, method or standard used (if necessary), qualitative and quantitative results of the controls carried out, declaration of conformity or not, follow-up of non- conformities, date or provisional period of the next quality control, etc.) |
| BPR-2-09: Identifying MD for Reform |
| It draws up and validates the planning of reform (decommissioning or withdrawal from service) with the care, medico-technical or technology-user departments and the directorates, while ensuring continuity of care |
| It provides, in partnership with the care or medico-technical services or technology users, for an unannounced reform procedure in the event of an incurable problem or too high a repair cost |
| The reformed (decommissioned) medical device, its accessories and possibly its specific consumables are visibly and explicitly marked and stored in an identified place, guaranteeing their non-use, pending their effective physical removal |
| Associated documentation is physically removed or archived in a specified location |


