All published articles of this journal are available on ScienceDirect.
Bone Defect Regeneration by a Combination of a β-Tricalcium Phosphate Scaffold and Bone Marrow Stromal Cells in a Non-Human Primate Model
Abstract
Background:
Reconstruction of large bone defects is a great challenge in orthopedic research. In the present study, we prepared composites of bone marrow-derived stromal cells (BMSCs) and β-tricalcium phosphate (β-TCP) with three novel aspects: proliferation of BMSCs with continuous dexamethasone treatment, cell loading under low pressure, and use of autologous plasma as the cell loading medium. The effectiveness of the resulting composite for large bone-defect reconstruction was tested in a non-human primate model, and the bone union capability of the regenerated bones was examined.
Materials and Methods:
Primary surgery: Bone defects (5 cm long) were created in the left femurs of nine cynomolgus monkeys with resection of the periosteum (five cases) or without resection (four cases), and porous β-TCP blocks were transplanted into the defects. Secondary surgery: Bone marrow aspirates harvested from seven of the nine monkeys were cultured with dexamethasone, and BMSCs were obtained. BMSCs were suspended in autologous plasma and introduced into a porous β-TCP block under low-pressure conditions. The BMSC/β-TCP composites were transplanted into bone defects created at the same sites as the primary surgery. Bone union evaluation: Five regenerated femurs were shortened by osteotomy surgery 8 to 15 months after transplantation of the β-TCP/BMSC composites, and bone union was evaluated radiographically.
Results:
After the primary surgery and treatment with β-TCP alone, one of the five periosteum-resected monkeys and two of the four periosteum-preserved monkeys exhibited successful bone reconstruction. In contrast, five of the seven cases treated with the β-TCP/MSC composite showed successful bone regeneration. In four of the five osteotomy cases, bone union was confirmed.
Conclusion:
We validated the effectiveness of a novel β-TCP/BMSC composite for large bone defect regeneration and confirmed the bone union capability of the regenerated bone.
INTRODUCTION
Autologous bone grafts, allografts and alternative biomaterials have been used to reconstruct large bone defects. Among them, autologous grafts have the highest osteogenic capability and sufficient mechanical strength, are free from virus transmission, and do not induce immune responses. However, the amount of autologous bone available for grafting is limited and the harvesting of bone grafts is associated with a relatively high risk of donor site morbidity, hematoma, and nerve injury [1-3]. Allografts offer osteoconductivity and mechanical strength at least immediately after surgery, and the use of allograft bone is unaffected by supply limitations. However, the characteristics of the allografts vary depending on the type of allograft and the preparation method. Furthermore, a relatively high complication rate has been reported for massive intercalary grafts in the treatment of osseous neoplasms, and for patients receiving adjuvant therapy, the survival rate of the allograft decreases to 50% within 10 years [4]. Synthetic calcium phosphate bonegraft substitutes composed of hydroxyapatite and/or tricalcium phosphates (TCPs) were developed to address these drawbacks,and the use of these materials is not hindered by the risk of virus transmission or limited availability. The osteogenic capacityof such grafts has been increased through continuous developmentof synthetic bone-graft substitutes; however,the osteogenic capabilityis still not sufficient for treating large bone defects.
Bone marrow-derived stromal cells (BMSCs) expanded by common culture protocols can differentiate into not only osteogenic cells but also chondrogenic, adipogenic, and other mesenchymal lineages [5]. Following the demonstration of bone formation by BMSCs, tissue engineering approaches using BMSCs have attracted attention for large bone-defect reconstruction [6]. Various studies have been conducted to augment the osteogenic capability of implants prepared using BMSCs to realize large bone-defect regeneration. However, satisfactory results have not been reported to date.
In this study, we present a combination of three modifications to the original concept of BMSC-based bone tissue engineering: culturing of BMSCs with continuous dexamethasone treatment [7] loading of cells into porous scaffolds under low pressure [8] and cell loading using autologous plasma as a cell loading medium [9]. Each of these modifications improved the bone formation capability by several fold compared with the conventional technique. Therefore, the total improvement achieved by the combined effect was greater than 10 fold. The aim of this study was to evaluate the effectiveness of porous β-TCP/BMSC hybrid implants prepared by combining these modifications for regeneration of large bone defects in a non-human primate model and to assess the resulting bone union capacity, which is an essential property for bone tissue and regenerated bone.
MATERIALS AND METHODS
All animal experiments were approved by the animal committee of Tokyo Medical and Dental University and were conducted according to the guidelines provided by the committee.
Study Design
A primary surgery was performed to assess the osteoconductivity of a porous β-TCP block without cells in a 5-cm-long femur bone defect. A secondary surgery was conducted to evaluate the effectiveness of β-TCP combined with BMSCs for regeneration of the large bone defect. To emphasize the effectiveness, the secondary surgery was conducted at the same site as the primary surgery, where the environment was considered to be more disadvantageous for bone regeneration than that of the native bone subjected to the primary surgery. In a third surgery, osteotomy of the femur was performed to evaluate the bone union capability of the regenerated bone.
Primary Surgery: Transplantation of β-TCP
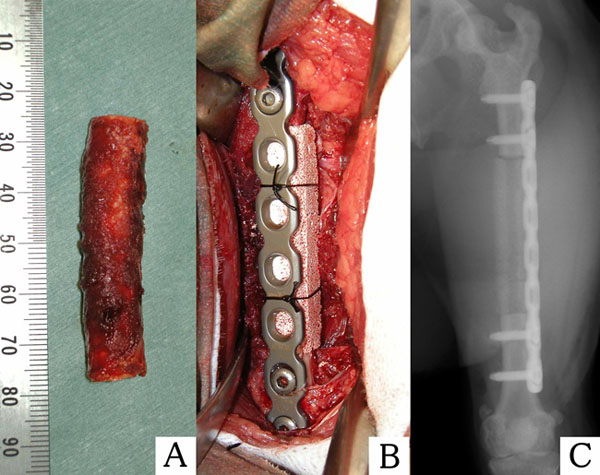
Nine skeletally mature cynomolgus monkeys (male, 5.7 to 7.2 kg) were used in this study and were divided into two groups: a periosteum-preserved group (n=4) and a periosteum-resected group (n=5). Under general anesthesia achieved by intramuscular administration of medetomidine hydrochloride (0.5 mg/kg) and ketamine hydrochloride (25 mg/kg), the animals were intubated, and general anesthesia was maintained with isoflurane. Each monkey was placed in the right lateral position, and the lateral side of the left femur was shaved. Following intramuscular injection of an antibiotic (cefazolin, 40mg/kg), the lateral aspect of the femur was sterilized with povidone iodine and covered with sterilized drapes, except for at the operative site. A 12-cm longitudinal incision was made at the lateral aspect of the femur, and the femur shaft was exposed by an intermuscular approach between the vastuslateralis and the biceps femoris. In the periosteum-preserved group, the periosteum was gently detached using a raspatrium, and a six-hole titanium alloy plate was contoured to the lateral aspect of the diaphysis and fixed with screws at the two proximal holes and two distal holes. A long bone segment (5 cm) was resected from the mid-diaphysis of the femur using a surgical saw. In the periosteum-resected group, when the resection site was approached, the thin layers of muscle surrounding the diaphysis were left attached to the femur to enable complete resection of the periosteum with the diaphysis. After plate fixation, the diaphysis, including the periosteum, was resected. In both groups, the resected segment was filled with hollowed porous β-TCP (Osferion®; Olympus, Tokyo, Japan). The specifications of the implant were as follows: porosity, 75%; dimensions, 1 x 1 x 5 cm; and diameter of the hollow, 3 mm. The implant was fixed to the plate by surgical nylon sutures, and the operative site was closed and sutured (Fig. 1). After surgery, the monkeys were allowed unlimited activity in the breeding cage.
Preparation of BMSCs
Bone marrow was harvested to obtain BMSCs 11 to 12 days prior to the secondary surgery. Under general anesthesia conducted as described above, 2.5-5.0 ml of bone marrow was aspirated from the bilateral iliac crest and the bilateral greater trochanters of the femurs of each macaque using a bone marrow biopsy needle (Cardinal Health, Dublin, OH) and combined with 30 ml of Dulbecco’s modified Eagle medium (DMEM; Sigma Chemical, St. Louis, MO) containing 200 IU of sodium heparin. The bone marrow was centrifuged (450 g, 5 min), and the bone marrow cell sediment was suspended in DMEM containing 10% fetal bovine serum, 1% antibiotic-antimycotic (10,000 units/ml penicillin G sodium, 10 mg/ml streptomycin sulfate and 0.025 mg/ml amphotericin B; Life Technologies, Carlsbad, CA, USA) and 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO). The bone marrow was plated in 225-cm2 culture flasks (BD Biosciences, Franklin Lakes, NJ) and cultured at 37°C in a humidified atmosphere with 5% CO2. The culture medium was replaced every 3 to 4 days, and nonadherent cells were removed. After 7 days of culture, attached BMSCs were detached with 0.25% trypsin containing 1 mM EDTA (Life Technologies) and reseeded in 225-cm2 culture flasks. The cells were detached and used for transplantation 4 to 5 days after subculture.
Preparation of the BMSC-Loaded Implant
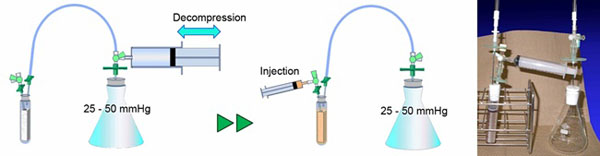
To prepare autologous plasma as a carrier for BMSCs, 18 ml of venous blood was collected from the femoral vein into a polypropylene tube containing 2 ml of citrate phosphate dextrose (CPD) solution (2.63% sodium citrate, 0.327% citric acid, 2.32% dextrose, and 0.251% sodium dihydrogenphosphate; TERUMO, Tokyo, Japan). The blood was then centrifuged at 2400 g for 15 min, and the supernatant was transferred to a new tube as autologous plasma and stored at room temperature until the following cell loading procedure. To load the BMSCs into the porous β-TCP scaffold, our original cell loading method was used, based on spontaneous penetration under low-pressure conditions and the use of an original apparatus (Fig. 2). Briefly, the porous scaffold was set in a tube, as shown in Fig. (2). and the air inside the apparatus was removed by suction using a 100 ml syringe until the pressure reached 25 to 50 mmHg. The detached BMSCs were suspended in 7 ml of autologous plasma and transferred to a 10 ml syringe. Immediately after 1 ml of 2% CaCl2 solution was added to initiate fibrin gel formation, the cell suspension was injected via a three-way stopcock into the bottom of the tube containing the scaffold. When the injection was complete, the tube was tapped to remove any bubbles attached to the scaffold, and the three-way stopcock was then released to recover normal pressure inside the apparatus. Within several minutes, gelation of the cell suspension was initiated, and the implant was withdrawn from the tube immediately before transplantation. The final cell concentration in the loaded fibrin gel was 1.3 x 106 to 4.1 x 106/ml.
Secondary Surgery: Transplantation of the BMSC-loaded Implant
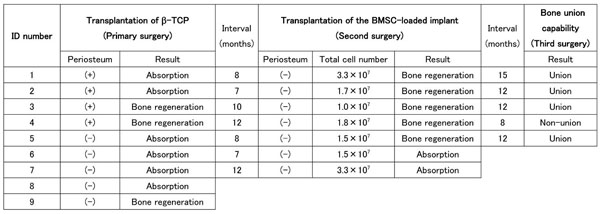
Seven of the nine macaques that had undergone primary surgery 7 to 12 months before hand under went a secondary surgery in which a β-TCP scaffold combined with BMSCs was transplanted into the same defect site as in the primary surgery (Table 1). The interval between the primary surgery and the secondary surgery depended on how long it had taken to confirm the results of the primary surgery, as the nature of the second surgery performed depended on the results of the first surgery. In cases of bone regeneration failure, the secondary surgery had to be performed before the atrophy of the remnant bone progressed, and in the case of successful bone regeneration, the second surgery was performed after the bone maturation was confirmed radiographically. In the case of successful bone regeneration by the primary surgery, the regenerated bone was resected with the surrounding periosteum-like tissue and replaced with the implant. In the case of bone regeneration failure following the primary surgery, the soft tissue and remnant bone tissue formed at the transplantation site were resected with the fascia of the surrounding muscles facing the transplantation site to remove the periosteum completely, and a bone stump with the normal bone marrow cavity exposed was formed using a steel burr before transplantation.
Third Surgery: Evaluation of the Bone Union Capability of the Regenerated Bone
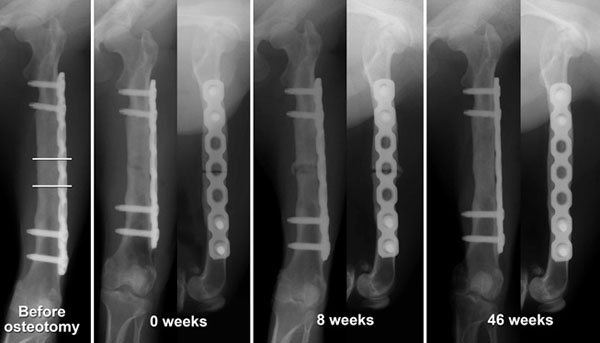
The bone union capability of the bone regenerated using the β-TCP/BMSC composite was evaluated in a femur osteotomy model. Five macaques whose femurs were successfully regenerated by the second surgery were included in this study 8 to 15 months after the second surgery (Table 1). Here, a 13-mm-long segment of the femur was resected from the center of the regenerated bone. The screws were removed from the two distal screw holes, and the plate was cut using a plate cutter at 13 mm from the distal end of the plate. The distal part of the femur was then fixed again at the two distal screw holes. Consequently, the femur was shortened, and the facing bone stumps were conjugated to the other because the interval between the screw holes was 13 mm. The resected bone segment was fixed in 10% neutral buffered formalin and used for histological evaluation.
Evaluation
For radiographic evaluation: antero-posterior and lateral X-ray images (60kV / 10mA / 0.3 sec) were collected at 2- to 4-week intervals under general anesthesia achieved by intramuscular administration of medetomidine hydrochloride (0.5 mg/kg) and ketamine hydrochloride (25 mg/kg), and bone regeneration and union were evaluated by two orthopaedic surgeons. For histological evaluation, the resected regenerated bone segments from the third surgery were fixed in formalin, decalcified with K-CX solution (Falma, Japan), dehydrated, embedded in paraffin, sectioned at a 5-μm thickness, and stained with hematoxylin and eosin.
Schedules of implant surgery, surgical conditions and the results of bone regeneration.
 |
RESULTS
Transplantation of Porous β-TCP Without BMSCs
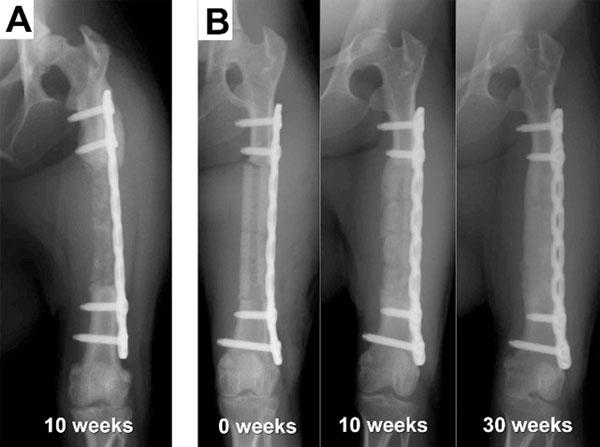
All of the animals, including those whose femurs were not regenerated, were able to walk and move freely throughout the experimental period, indicating that the plate fixation provided sufficient strength. In the periosteum-preserved group, two cases showed bone regeneration throughout the defect site, whereas the other two cases showed only partial and intermittent bone formation, and most of the transplanted β-TCP was resorbed within 12 weeks. In the periosteum-resected group, one case unexpectedly showed complete bone regeneration (Fig. 3B, Table 1), whereas four of the five cases failed to achieve bone regeneration, as predicted (Fig. 3A, Table 1). In the bone-regenerated cases, bone formation appeared to occur not only from the osteotomy stumps but also evenly throughout the transplanted site and surrounding area, indicating that bone-forming osteoblasts originated from not only the adjacent bone marrow but also the surrounding periosteum or muscle tissue.
Transplantation of the Porous β-TCP/BMSC Hybrid
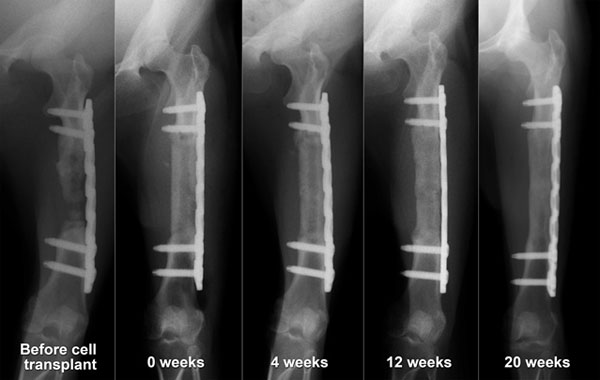
Regardless of the results of the primary surgery, all four macaques in the periosteum-preserved group and three macaques in the periosteum-resected group were used for evaluation of the BMSC-loaded implant. Five of the seven cases showed successful bone regeneration (Fig. 4, Table 1), and two cases resulted in failure. A statistical comparison with the control without BMSCs was not performed because the number of animals was small and because the environment at the transplanted sites was different from that at the sites of the first surgery. There was no correlation between the bone regeneration results and the transplanted cell number.
Bone Union Capability of the Regenerated Femurs
Five regenerated femurs were tested for their bone union capability, which is one of the most important characteristics of natural living bone. Four of the five shortened regenerated bones showed bone union that could easily be identified from X-ray images (Fig. 5, Table 1). In the case of bone union failure, the gap between the bone stumps was relatively large (1.5 to 2.0 mm) because of poor surgical technique, and a higher amount of β-TCP remnants was recognized in the resected bone tissue by macroscopic observation compared with the other cases (Fig. 6B).
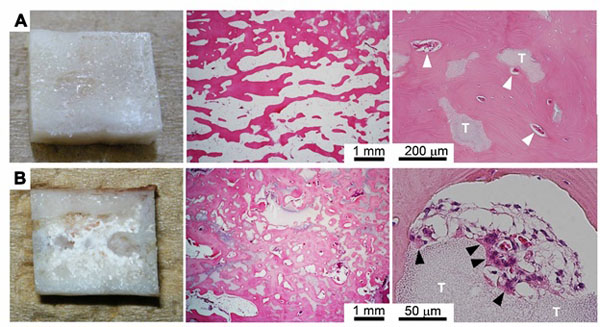
Histology
The center portion of the regenerated bone resected in the third surgery was used for histological evaluation. The bone tissue on the medial side of the regenerated femur was dense and matured nearly equally to normal cortical bone. In contrast, the bone tissue on the titanium plate side was not as dense and mature as that on the medial side because of stress shielding. In contrast to the normal diaphysis, which consists of a hollow cortical bone and fatty marrow that fills the cavity, most of the regenerated bone was cancellous bone-like tissue exhibiting blood vessels and incorporated β-TCP remnants (Fig. 6A). The regenerated bone in the case with union failure included more abundant β-TCP remnants, as also determined by macroscopic observation. However, more osteoclast-like multinucleated cells were attached to the remnants. Therefore, the abundance of remnants would have likely decreased if harvesting had been conducted at a later time point, and bone union may have been achieved.
DISCUSSION
In this study, we succeeded in the regeneration of 5 cm long bone defects using porous β-TCP blocks with or without BMSCs in a non-human primate model. The success rate of the β-TCP/BMSC hybrid was higher than that of β-TCP alone, although a statistical analysis was not performed. The regenerated bone exhibited bone union capability, which is one of the most important characteristics of natural living bone.
The reconstruction of large bone defects caused by bone tumors, trauma, and infection is a major challenge in the orthopedic field. Many studies have attempted the reconstruction of large bone defect, and large animal models have been used in such studies to verify the practicality of the results. Mastrogiacomo et al. reported the osteoconductivity of a scaffold composed of hydroxyapatite and silicon-stabilized TCP in a 4.8 cm long tibia defect model [10] and then reported enhanced osteoconductivity when using the same scaffold combined with BMSCs ina 4.5 cm tibia defect model [11]. These findings indicate the insufficiency of scaffold transplantation alone and the efficacy of scaffold-BMSC transplantation for large bone defect reconstruction. Other reports have also demonstrated the effectiveness of BMSCs for reconstruction of large bone defects [12-15].
To augment bone formation by BMSCs using the conventional method developed by Caplan et al. [6] various methods, such as gene transfer and perfusion culture, have been attempted. In the present study, we implemented three modifications to the conventional method to facilitate large scale bone regeneration.
The first modification involved optimization of the cell culture method to obtain BMSCs with a higher differentiation capability. A popular osteogenic induction protocol for BMSCs uses dexamethasone only during the induction of differentiation with ascorbic acid phosphate and β-glycerophosphate. In contrast to the standard protocol, the culture protocol used in this study used dexamethasone during the entire BMSC proliferation period. A previous study demonstrated that human BMSCs proliferated in dexamethasone-containing medium presented higher enhanced differentiation not only into the osteogenic lineage but also into the chondrogenic and adipogenic lineages, and furthermore that the bone formation capability of macaque BMSCs that proliferated in the presence of dexamethasone was at least two-fold higher than that in control medium [7].
The second modification that we applied was cell loading under a low pressure system. To efficiently infiltrate liquid into porous materials, it is important to remove the air inside the pores; therefore, low pressure has been generally applied after immersion of the material to remove residual air. However, the complete removal of residual air from the immersed porous material is prevented by the surface tension of the surrounding liquid. In the present study, low pressure was thus applied to the porous scaffolds before immersion in the cell suspension to more efficiently remove the air from the porous materials. Using 5 mm cubic, porous β-TCP blocks and a heterotopic bone formation model with rat BMSCs, previous studies demonstrated that the cell loading efficiency and bone formation of hybrid implants prepared under the low pressure cell loading method were increased by 2 and 2.5 fold, respectively, compared with preparation by removal of air after immersion [8].
The third modification that we applied was related to the cell loading medium. When BMSCs were introduced into the porous scaffold, we used autologous blood plasma as a cell suspension medium; in contrast, BMSCs are suspended in the culture medium in conventional methods. Because the introduced plasma formed a fibrin gel within minutes in the pores of the porous scaffolds and the BMSCs were firmly maintained within the gel, the implant could be transplanted without waiting for cell attachment to the scaffold. Thus, this method would be convenient for use in the clinic; furthermore, preparation of BMSCs in this manner has been shown to augment bone formation in heterotopic bone formation models. In particular, for bone formation using a macaque heterotopic bone formation model, it was shown that a hybrid implant of a porous scaffold and BMSCs prepared using autologous plasma required one-tenth of the number of cells needed when culture medium was used as the cell loading medium [9]. Thus, BMSCs suspended in plasma showed a ten-fold increase in bone formation compared to the same number of BMSCs prepared using conventional techniques.
The combination of these modifications should greatly augment bone formation capability relative to the conventional method and contribute to the regeneration of 5 cm long bone defects. However, in the present study, some cases of control implants that did not receive BMSCs also achieved bone regeneration, and in such cases, bone formation did not necessarily occur from the femur stumps. Recently, intramuscular osteoinduction by biomaterials has been reported [16-22] and this osteoinduction was associated with two common factors: the use of a large-animal model, such as baboons, dogs, or sheep [22] and the use of a material with a macroporous or microporous structure to increase the specific surface area [19, 21]. The present study used a macaque large-animal model, and the porous β-TCP had macropores and micropores.The findings reported herein demonstrate that porous β-TCP has strong osteoconductivity and possibly osteoinductivity, and that β-TCP can function as a fourth factor in addition to the three modifications we made to the BMSC preparation protocol to augment bone formation in large bone defects.
This study demonstrated the efficacy of β-TCP/BMSC hybrid transplantation for 5 cm long critical-size bone defects using a non-human primate model and revealed the bone union capability of the regenerated bone. However, two of the seven BMSC transplanted cases failed to show bone regeneration, and the extent of regeneration was not correlated with the transplanted cell number. This finding suggests that the quality of the BMSCs used for transplantation is more important than the number of transplanted BMSCs as reported in the literature [23]. Criteria for appropriate preparation of BMSCs for transplantation should be established for safe and reliable BMSC based clinical bone regeneration.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We also acknowledge Olympus Co. for kindly donating the β-TCP ceramic blocks.


