RESEARCH ARTICLE
Enzymatic Detachment of Therapeutic Mesenchymal Stromal Cells Grown on Glass Carriers in a Bioreactor
Denise Salzig1, *, Alexandra Schmiermund1, Pablo P. Grace1, Christiane Elseberg1, Christian Weber1, Peter Czermak1, 2, 3
Article Information
Identifiers and Pagination:
Year: 2013Volume: 7
First Page: 147
Last Page: 158
Publisher ID: TOBEJ-7-147
DOI: 10.2174/1874120701307010147
Article History:
Received Date: 19/9/2013Revision Received Date: 11/11/2013
Acceptance Date: 11/12/2013
Electronic publication date: 27 /12/2013
Collection year: 2013

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
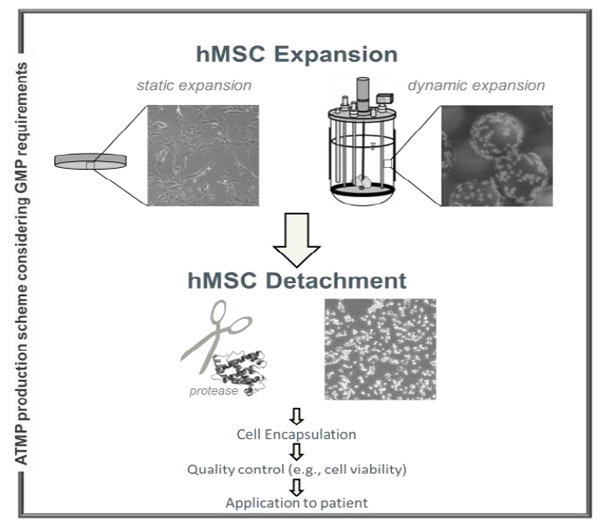
Cell therapies require the in vitro expansion of adherent cells such as mesenchymal stromal cells (hMSCs) in bioreactor systems or other culture environments, followed by cell harvest. As hMSCs are strictly adherent cells, cell harvest requires cell detachment. The use of hMSCs for cell therapy requires GMP production in accordance with the guidelines for advanced therapeutic medical products. Therefore, several GMP-conform available proteolytic enzymes were investigated for their ability to promote hMSC detachment. An allogeneic hMSC cell line (hMSC-TERT) that is used in clinical trials in the form of alginate cell capsules was chosen as a model. This study investigated the influence of several factors on the outcome of proteolytic hMSC-TERT detachment. Therefore, hMSC-TERT detachment was analyzed in different cultivation systems (static, dynamic) and in combination with further cell processing including encapsulation. Only two of the commercially available enzymes (AccutaseTM, TrypZeanTM) that fulfill all process requirements (commercial availability, cost, GMP conditions during manufacturing and non-animal origin) are found to be generally suitable for detaching hMSC-TERT. Combining cell detachment with encapsulation demonstrated a high impact of the experimental set up on cell damage. It was preferable to reduce the temperature during detachment and limit the detachment time to a maximum of 20 minutes. Cell detachment in static systems was not comparable with detachment in dynamic systems. Detachment yields in dynamic systems were lower and cell damage was higher for the same experimental conditions. Finally, only TrypZeanTM seemed to be suitable for the detachment of hMSC-TERT from dynamic reactor systems.